Using cell reprogramming, so-called induced pluripotent stem cells (iPS cells) can be generated from a blood or skin sample. The body cells are reset into an embryonic stage and are then able to differentiate further into a huge variety of cell types again such as heart muscle or brain cells. The expectations of these all-rounders are accordingly high. “Nerve cells produced from iPS cells are nowadays the most attractive tool for research into brain diseases and pharmaceutical research,” said Prof. Dr. Oliver Brüstle from the Institute of Reconstructive Neurobiology at the University Hospital Bonn (UKB).
Such human nerve cells derived from iPS cells can vary considerably. Depending on the cell culture method and production route chosen, they react very differently in experiments. “However, we are looking for a cell model that is able to produce the same results when an experiment is repeated,” explains Dr. Michael Peitz from Brüstle's team. After all, the results of the studies should be statistically verified.
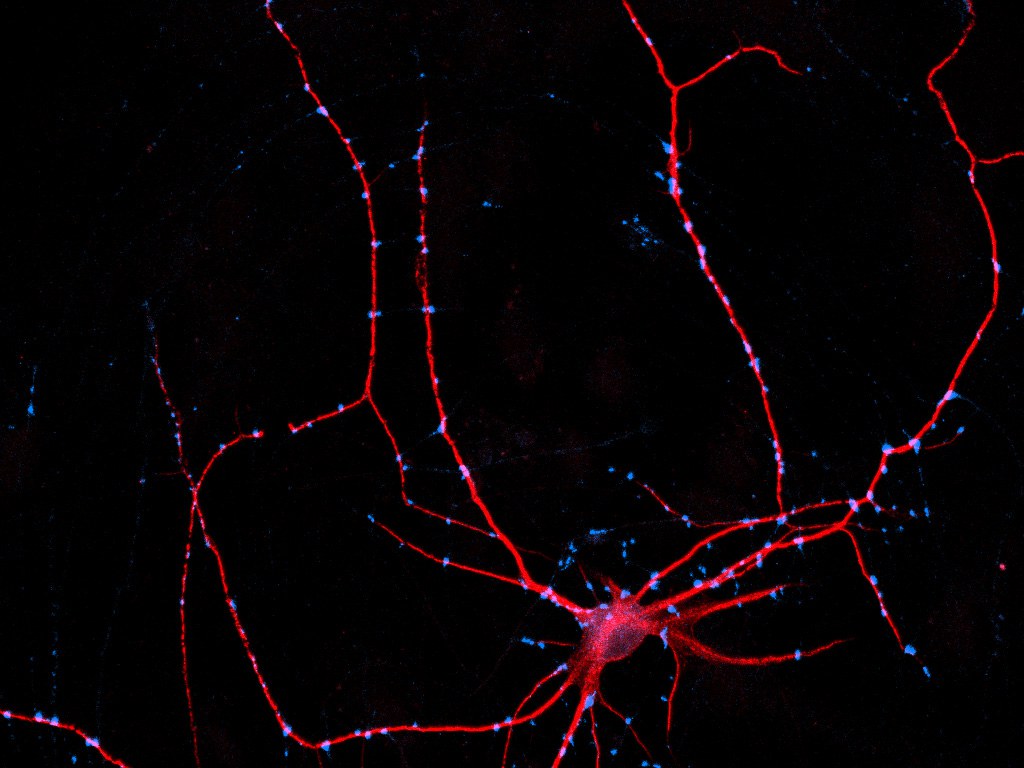
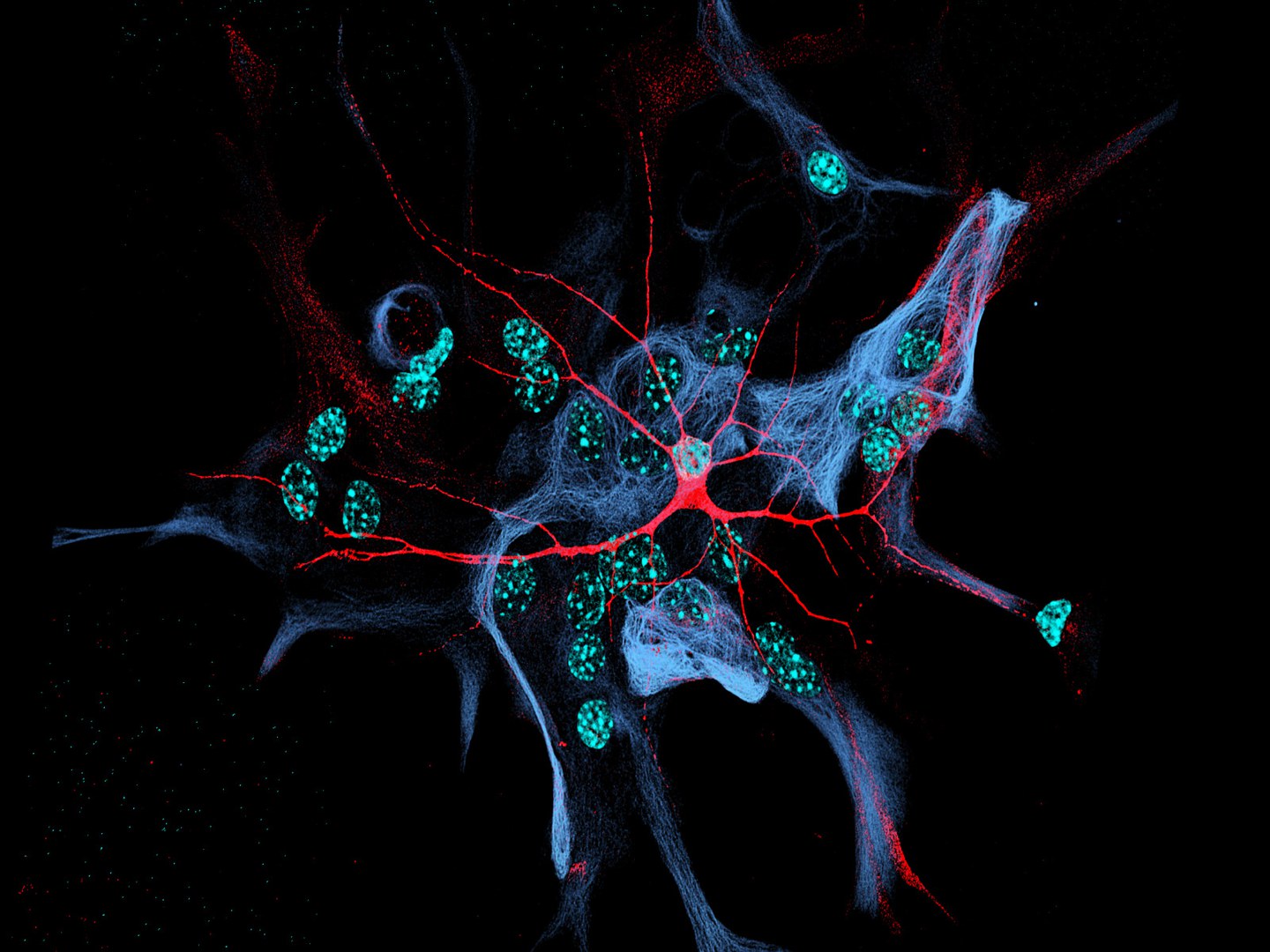
For this reason, the UKB scientists, together with the Max Planck Institute (MPI) for Experimental Medicine in Göttingen and the Vrije Universiteit Amsterdam, developed and tested a cell culture model consisting of a single nerve cell obtained from human iPS cells via a highly standardized cell programming method. This “single” sits on glial cells, which are natural neighbors of nerve cells and crucial for their maintenance and function.
The nerve cell is talking to itself
The special feature: The “single” brain cells talks to itself because its main nerve fiber (axon) ends up connecting to processes of the same nerve cell. “In principle, it’s a single neurons with a short-circuit,” explains Dr. Kristina Rehbach, one of the lead authors of the two studies at the Institute of Reconstructive Neurobiology at the UKB. This allows the scientists to eavesdrop on the “single” nerve cell chatting with itself.
The circular signal transmission between the axon and the respective neuron takes place via synapses. These are interfaces at which electrical signals cause the release of messenger substances, which again lead to electrical impulses on the receiver side. Here the signals can be amplified or reduced. The scientists at the MPI in Göttingen and the Vrije Universiteit Amsterdam tested how this single-cell model behaves in stimulation experiments. They used both neurons responsible for excitation in the brain as well as inhibitory nerve cells. “We were able to demonstrate that this model, which consists of only a single nerve cell, yields highly reproducible data in the functional tests and thus represents a very good basis for high-throughput experiments,” says Prof. Dr. Matthijs Verhage from the Vrije Universiteit Amsterdam.
Various applications
The research team sees many possible applications for the “single” nerve cell model. It can be used to study disease mechanisms. “For example, if a protein at a synapse is altered by a gene mutation, the consequences for signal transmission can be observed directly in this model,” said Prof. Brüstle. Another advantage is that iPS cells reprogrammed from the skin or blood of patients can be used to generate neurons with disease- and patient-specific features. The cell model could of particular interest for pharmaceutical research because it is standardized, scalable and applicable to a wide variety of brain diseases.
“The excellent cooperation of the various research teams in this project shows that the combination of stem cell technology and functional synapse biology opens up entirely new perspectives,” says Prof. Dr. Jeong Seop Rhee from the MPI for Experimental Medicine in Göttingen. All three research teams work together in the European joint project COSYN.
Publications:
A Single-Cell Model for Synaptic Transmission and Plasticity in Human iPSC-Derived Neurons, Cell Reports, DOI: 10.1016/j.celrep.2019.04.058
An Autaptic Culture System for Standardized Analyses of iPSC-Derived Human Neurons, Cell Reports, DOI: 10.1016/j.celrep.2019.04.059
Media contact:
Prof. Dr. Oliver Brüstle
Institute of Reconstructive Neurobiology, University Hospital Bonn (UKB)
Tel. +49 (0)228-6885 500
E-mail: r.neuro@uni-bonn.de