The cell nucleus is a kind of vault: It is located in the cell and guards the DNA, which contains the building instructions for all the cell's proteins. When the cell needs a protein for a specific task, it orders a transcript of the matching DNA segment in the nucleus. This copy leaves the nucleus and reaches the ribosomes, complex molecular machines. These then work through the instructions step by step to produce the desired protein.
This means that the vast majority of cell molecules are produced outside the nucleus. However, this does not apply to the ribosomes themselves: Their numerous components are already largely assembled inside the nucleus. This results in the formation of two large molecular complexes, the pre-60S and the pre-40S subunits. Both are then passed through the nuclear pores into the cell, where they are assembled in a final step to form the ribosome.
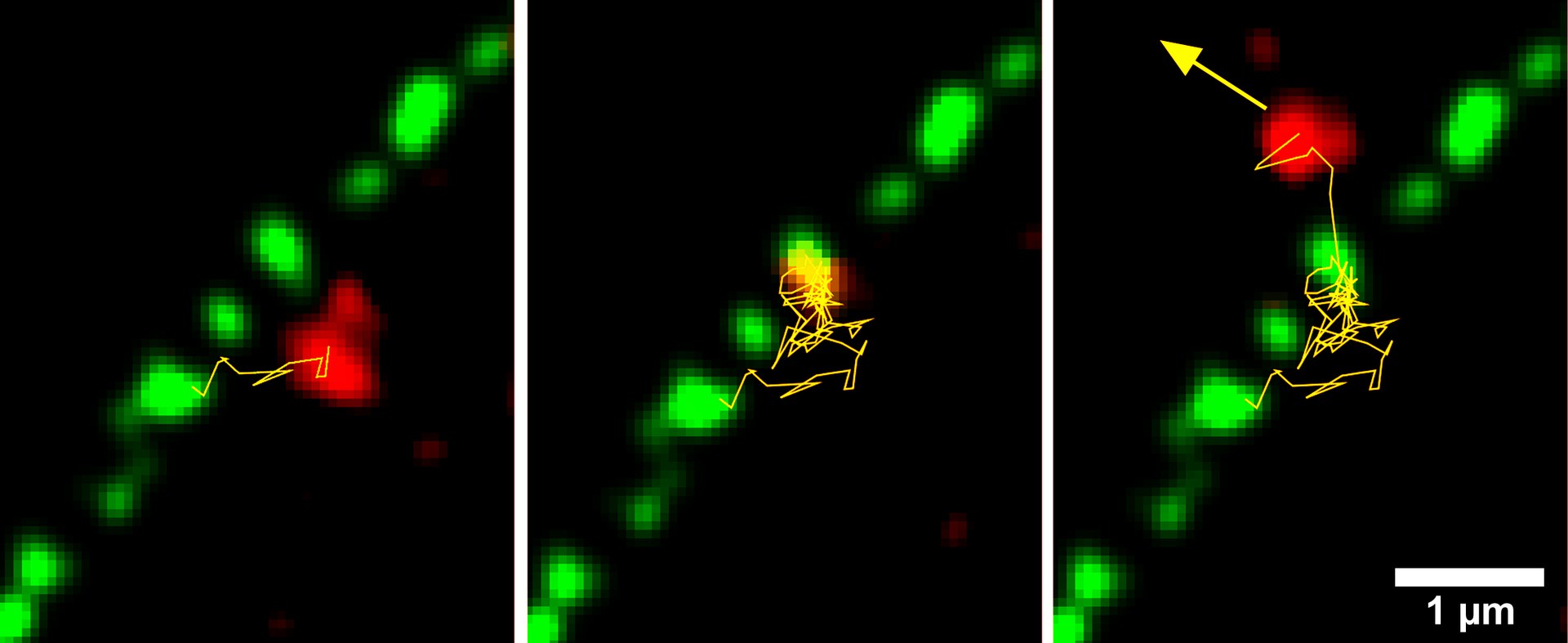
The team from the University of Bonn and ETH Zurich has now filmed exactly how the export of the larger pre-60S subunit takes place. "To do this, we stained the nuclear pores with a green dye and the pre-60S unit in red," explains Prof. Dr. Ulrich Kubitscheck of the Institute for Physical and Theoretical Chemistry at the University of Bonn. The recording itself was done with the help of a special microscope, which the researchers had also specifically modified for this purpose.
Gel plugs as pore stoppers
"In this way, we have succeeded for the first time worldwide in capturing the passage of individual ribosome components through a pore in real time," says the study's lead author Dr. Jan Ruland, who completed his doctorate in Kubitscheck's research group. This is anything but trivial: The nucleus of human cells is virtually covered with several thousand pores. Each of them has a diameter of only about one ten-thousandth of a millimeter. The success is based on advances in microscopy technology, but also on more than ten years of research work in which the researchers have continuously optimized their method.
Transport through the pores is a complex process: They are sealed with a type of gel that normally prevents the passage of larger molecules. The subunits of ribosomes are huge; without helpers, they could not leave the nucleus. They therefore surround themselves with certain molecules, the export receptors. This allows them to "swim through" the gel plug, so to speak. "On the outside of each pore sits a protein gripper that pulls out the ribosome unit," explains Kubitscheck's colleague Dr. Jan Peter Siebrasse.
Passage takes only 25 milliseconds
This step seems to be the "bottleneck" of the transport process. "We were able to demonstrate that the pre-60S units pile up exactly at the point where the protein gripper reaches into the pore," says Siebrasse, who, like Kubitscheck, is a member of the Transdisciplinary Research Area "Building Blocks of Matter and Fundamental Interactions" (TRA Matter). Nevertheless, the export proceeds relatively quickly - the study participants estimate that 35 to 50 subunits per second can pass through a single pore.
But the evaluation of the films also shows that the export does not always work. Only in every third case in which a pre-60S unit came into contact with a pore did it then actually exit the nucleus. In the remaining cases, however, the process was aborted - possibly because other molecules were being transported out of the nucleus at the same time, Kubitscheck speculates.
The study provides more detailed insights into the formation of ribosomes. In addition, the method is also suitable for investigating other transport processes. "We have pretty much exhausted the current technical possibilities," says Kubitscheck. "It's not without reason that the reviewers have called our study a reference that we hope other groups can benefit from."
Participating institutions and funding:
Researchers from ETH Zurich were also significantly involved in the study. The work was supported by the University of Bonn and the Swiss National Science Foundation.
Publication: Jan Andreas Ruland, Annika Marie Krüger, Kerstin Dörner, Rohan Bhatia, Sabine Wirths, Daniel Poetes, Ulrike Kutay, Jan Peter Siebrasse and Ulrich Kubitscheck: Nuclear export of the pre-60S ribosomal subunit through single nuclear pores observed in real time; Nature Communications; DOI: 10.1038/s41467-021-26323-7
Offer for TV journalists:
Television crews are welcome to get a picture of the method on site and document the experiment on film.
Contact:
Prof. Dr. Ulrich Kubitscheck
Institute for Physical and Theoretical Chemistry
University of Bonn
Phone +49-228-732262
E-Mail: u.kubitscheck@uni-bonn.de