Both, animals and plants evolved particular barrier systems that limit the uncontrolled diffusion of compounds to allow selective nutrient uptake and balance. Intestinal absorptive cells in the animal gut are connected by so-called “tight junctions” which seal the intercellular spaces. Thus, membranes of the absorptive cells determine which nutrients are taken up in the gut. The composition of the intestinal flora, resident microbes in the gut, influences the intestinal barriers function. Mechanistically similar, barriers in plant roots are localized in an inner cell layer called the endodermis.
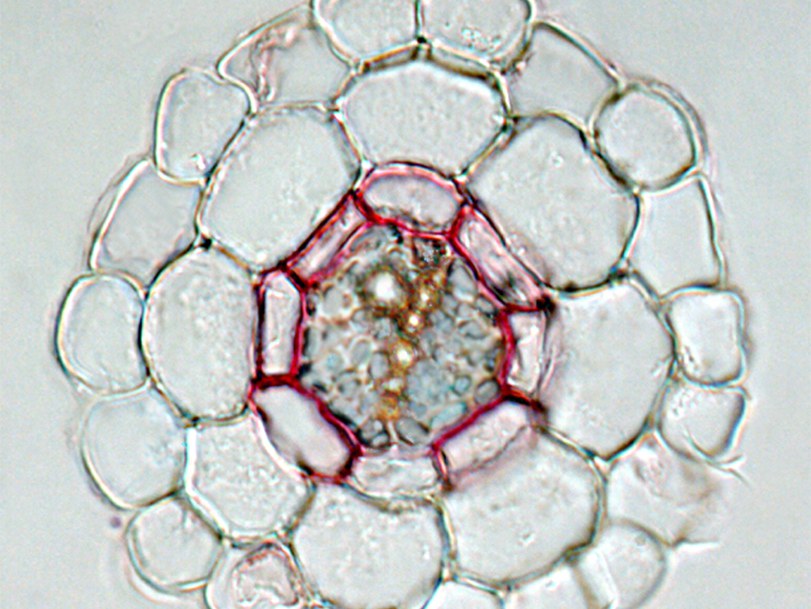
In plants, two types of barriers exist in the endodermis: Casparian strips, named after the discoverer Robert Caspary who was director of the herbarium and adjunct head of the Bonn Botanical Garden in the 1850s. Casparian strips are made of lignin and form a fine ring that encircles the endodermal cells and seals the spaces between adjacent endodermal cells. Suberin depositions are cork-like biopolymers forming an insulating layer in the cell wall which encases the entire surface of the endodermal cell. These barriers facilitate transport processes regulated by the endodermal cells to control which minerals and compounds are taken up into the plant or released towards the outside into the soil. Whether and how root barrier function coordinates with microorganism in the soil is unknown.
The researchers exploited a collection of root barrier mutants in the model plant Arabidopsis thaliana to investigate if the extent and integrity of root barriers affects the structure of the root microbiota. Conversely the team surveyed 416 individual bacterial strains isolated from soil for their ability to manipulate the function of Casparian strips and suberin depositions. Furthermore, they designed and deployed bacterial synthetic communities of 41 representative strains to uncover the molecular basis of these interactions. Likewise, the researcher deployed the synthetic communities to analyze the impact of microorganisms on plant mineral nutrient homeostasis, growth and performance under deficient conditions.
These studies showed that the properties and function of the root barriers also influences the composition of the root microbiota. Conversely, individual members of the plant microbiota or bacterial communities have the competence to alter the development and function of endodermal barriers, especially the suberin depositions which are critical for the mineral transport across the root. Intriguingly, this relationship is based on the competence of the bacteria to modify the effect of the phytohormone abscisic acid, which on his part can act on suberin synthesis. Ultimately, the bacterial communities controlled via abscisic acid which minerals accumulated in the plant or were depleted. Interestingly, the microbiota mediated modification of the suberin barriers also provoked a better adaptation to nutrient deficiency and stress conditions. Plants associated with the synthetic bacterial communities grew significantly better under phosphate and zinc depletion, and also in salt stress conditions, as compared to plants without bacteria.
„These discovery has potential applications in plant nutrition and agriculture“ says coauthor and adjunct professor Dr. Rochus Franke from the Institute of Cellular and Molecular Botany (IZMB). Shaping root microbiota may represent a new and faster strategy to increase crop yield, beneficial mineral nutrient contents and cultivate more resilient crops, compared to longtime breeding efforts. Dr. Franke is convinced that „in the long term microbial-based approaches can be used to improve food production and food quality”.
Publication: Salas-Gonza´lez et al.: Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis, Science 371, Internet: https://doi.org/10.1126/science.abd06951
Contact:
Privatdozent Dr. Rochus B. Franke
Institute of Cellular and Molecular Botany (IZMB)
University of Bonn
Tel. +49-(0)228/736525
E-mail: rochus.franke@uni-bonn.de